Theme: Probiotics for Human Health: New Innovations and Emerging Trends
Probiotics 2016
ConferenceSeries Ltd welcomes and invites all the participants from over the world to attend The 5th International Conference and Exhibition on Probiotics, Functional and Baby Foods to be held on November 14-16, at Orlando, USA.
ConferenceSeries Ltd is the only scientific organization dedicated specifically to probiotics, bringing together scientists from all pertinent disciplines, including food science, microbiology, immunology, biochemistry, nutrition, molecular biology and medicine.
Probiotics 2016 highlights the theme “Probiotics for Human Health: New Innovations and Emerging Trends.” Probiotics 2016 event is designed in a way that it provides all the participants a platform to expand their ideas on recent advances made in the science of probiotics, highlighting their current and future roles in maintaining health and preventing diseases. Facilitating a global co-operation between scientists and institutions is another benefit. Probiotics 2016 will be a landmark event for the establishment of collaborations and direct knowledge distribution among doctors, physicians, business delegates, expert professionals and academicians, scientists and researchers exploring the field of Probiotics, Prebiotics, Food Technology and Nutrition.
Why you should attend Probiotics 2016 International conference?
This scientific conference will bring an opportunity to share potential benefits, applications and challenges followed by future opportunities of probiotics in preventing and controlling a wide range of diseases affecting adults, infants, animals and environment thereby escalating the quality and endurance of life. Furthermore, participants will get an opportunity to meet those who have made a positive impact on the past as well as influenced the present and notably those who will alter the future of probiotics by means of regulatory efforts, clinical trials, basic research, or development of industrial technology.
Target Audience:
- Young and brilliant researchers
- Doctors
- Professionals
- Directors
- CEO’s
- Presidents
- Vice-presidents
- Professors
- Associate professors
- Assistant professors
- Dieticians
- Business Delegates from allied industries and companies
Probiotics – 2016
Theme: Probiotics for Human Health: New Innovations and Emerging Trends
Summary:
Probiotics are live microorganisms which are found naturally in our body and are good for our health, especially our digestive system. Many types of bacteria are classified as probiotics, however, the most common are bacteria that belong to groups called Lactobacillus and Bifidobacterium and in case of yeast it is Saccharomyces boulardii. Probiotics works as a supplement for the “good” bacteria in our body, thereby maintaining the balance between the “good” and “bad” bacteria which allows our body to work normally. Some common conditions they treat are digestive disorders, allergic disorders, common cold, oral health problems, colic in infants, and prevention of necrotizing enterocolitis in very low birth weight infants. Products sold as probiotics include foods, dietary supplements, and products that are not used orally, such as skin creams. Popular probiotic products include Herbalife Activated Fiber, Amway Nutrilite Fiber, HealthAid Acidophilus plus 4 Billion, Zenith Nutrition Probiotic Immune etc.
For more details please visit: http://probiotics.conferenceseries.com/
Importance and Scope:
Probiotics 2016 accentuates the theme “Probiotics for Human Health: New Innovations and Emerging Trends”. Probiotics 2016 event is devised in such a way that it caters an exclusive platform for new researchers, scholars, students and educators to present, discuss, share and learn about the most recent trends, innovations and concerns, practical challenges encountered and the solutions adopted for them as well as helps you network with new people in the concerned field. The two days of educational program will include keynote presentations, session speakers, and poster presenters on the latest pioneering techniques as well as papers in the areas of probiotics in aquaculture, food technology, diary technology, veterinary medicine, microbial fermentation, health benefits and novel applications of probiotics.
Surge in the functional food demand is expected to benefit the overall probiotics marke trends. This concept is gaining popularity because consumers are getting aware of the link between health, diet and nutrition. Their ability to improve the health of consumers by strengthening their immune system either directly or indirectly by improving the condition of the gut, digestive system, and nutritional value is expected to benefit the overall probiotic ingredient market growth.
Probiotics Market size was valued at USD 36.6 billion in 2015, with over 7% CAGR growth expectation from 2016 to 2023. This latest global probiotic market report predicts revenue in ingredient sales to exceed USD 64 billion by 2023. Global functional food market was valued at USD 19.24 billion in 2007, growing to over USD 31 billion in 2014, with health benefits offered beyond the traditional nutrition function. Functional foods have steadily gained acceptance, driven by the rising prevalence of lifestyle diseases.
Guts are 70% responsible for keeping the immune system strong, and keeping the body fit, and reducing obesity. These ingredients help in treating diseases such as intestinal inflammation, urogenital infections, and antibiotic associated diarrhea by fighting against bad bacteria in the guts.
In 2015, yogurts accounted for over 57% of the probiotic dairy products industry revenue and over 50% of the global probiotics market size. Growing gut health awareness has been driving per capita expenditure on these ingredients across key regions. Prevalence of digestive disorders in Japan, the U.S., and Western Europe will benefit demand.
Probiotics Market
North America, with U.S. probiotics market size accounts for over 84% regional revenue in 2015, will likely see moderate gains, as participants struggle to meet regulatory and penetration challenges. Europe probiotics market share will gain by 2023, with a supportive political landscape. Favorable regulatory framework set up by severalorganizations such as Scientific Committee on Animal Nutrition, European Food Safety Authority and Novel Food Regulation are expected to aid regional demand. Germany & UK are largest European industries. Dietary supplements are highly prevalent in Europe, owing to growing health issues such as obesity and digestive disorders stemming from unhealthy and sedentary lifestyle. APAC, driven by China & Japan probiotics market size, will remain the largest regional industry for participants. While Japan will continue to remain a country forefront on probiotic ingredient consumption, China and India are likely to see maximum growth. LATAM and MEA are relatively underdeveloped regions, and may see industry development throughout the forecast timeframe.
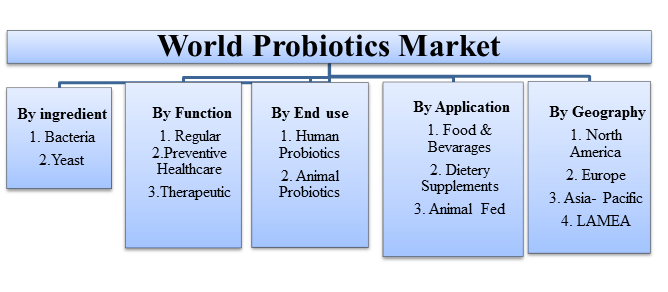
Figure-1: World Probiotcs Market
Global Probiotics Application Outlook (Revenue, USD Million, 2012 - 2020)
• Probiotic Food and Beverages
• Probiotic Dietary Supplements
• Animal Feed Probiotics
Global Probiotics End-Use Outlook (Revenue, USD Million, 2012 - 2020)
• Human Probiotics
• Animal Probiotics
Global Probiotics Regional Outlook (Revenue, USD Million, 2012 - 2020)
• North America
• Europe
• Asia Pacific
• RoW
Competitive Market Share:
Global probiotics market share is highly fragmented and the top five business players accounted for less than 40% of the total revenue in 2015. Danone accounted for over 15%, followed by Yakult Honsha. Although the industry is growing at a significant pace, development and innovation are heavily dependent on scientific substantiation.
Food companies will continue to research new food products, resulting in association of more food ingredients with health claims. In 2012, Chr. Hansen launched a probiotic solution which can help tackle diarrhea by shortening the duration of diarrhea and prevent dehydration.
APAC is the fastest growing region in the probiotics market with the maximum demand arising from Japan.
The increasing product innovations in the probiotic market is also expected to fuel the market growth. New probiotic products are being introduced in the form of chocolate, biscuits, and gums to pique the interest of the customer. For instance, during 2014, Milsling introduced a probiotic dark chocolate brand named 'Chocowise'. The brand is sold in numerous markets such as Serbia, Croatia and Bosnia and is expected to enter other European markets as well.
Key players operating in the probiotics market include BioGaia AB, Danone, Chr. Hansen Holding A/S, Yakult Honsha Co. Ltd., Probi AB, Lifeway Foods, Inc., Nestle S.A., Ganeden, Inc., E. I. du Pont de Nemours and Company, and Protexin.
Why Orlando?
Orlando is a city in the U.S. state of Florida, located in Central Florida, it is the centre of the Orlando metropolitan area, which had a population of 2,387,138, according to U.S. Census Bureau figures released in March 2016, making it the 24th largest metropolitan area.
The City of Orlando is nicknamed "The City Beautiful" and its symbol is the fountain at Lake Eola. Orlando is also known as "The Theme Park Capital of the World" and in 2014 its tourist attractions and events drew more than 62 million visitors. The Orlando International Airport is the thirteenth busiest airport in the United States and the 29th busiest in the world. Orlando's famous attractions form the backbone of its tourism industry: Walt Disney World, the Universal Orlando Resort, SeaWorld, Gatorland and Wet 'n Wild waterpark. The city is also one of the busiest American cities for conferences and conventions.
Orlando is a major industrial and hi-tech center. Orlando has the 7th largest research park in the country, Central Florida Research Park, with over 1,025 acres (4.15 km2). Orlando's fertile farmlands, regional healthcare system, and expertise in photonics and MS&T have given rise to a strong biotechnology industry. More than 500 biotechnology and life sciences companies earn $3.6 billion each year in such areas as research, clinical trials, agricultural sciences, and medical training. This vibrant field has applications in industrial food ingredients, plant reproduction, bioterrorism defence, medical products, and modeling systems for laboratories.
List of Hospitals Research Centre:
Orlando, USA
- Nemours. Children’s Health System
- Orlando Clinical Research Centre
- Florida Hospital Orlando
- Orlando Health
- Arnold Palmer Hospital for Children
Worldwide
- MRC Human Nutrition Research
- UK HealthCare
- Nutrition Australia
- Great Ormond Street Hospital for Children
- Bedford Hospital
- Human Nutrition Research Centre
- Children’s Nutrition Research Centre
- Nutrition Studies Research Group
Major Probiotic Associations and Society:
- International Probiotic Association
- International Scientific Association for Probiotics and Prebiotics
- Probiotic Association of India
- European Probiotic Association
- International Probiotic Association Europe
- American Nutrition Association
- American Pregnancy Association
- Global Alliance for Probiotics
- Academy of Nutrition and Dietetics
- American society of nutrition
- British Dietetic Association
- Society of Nutrition Education
Companies Associated with Probiotics:
- AB-biotics Bellaterra (Barcelona), Catalonia, Spai
- Atrium Innovations Montreal, Canada
- Bayer Basel, Switzerland
- BIO-CAT Microbials Shakopee, MN, USA
- Bifodan Hundested, Denmark
- BIOSEARCH Granada, Andalusia, Spain
- Biocare Copenhagen, Denmark
- BLIS Technologies Ltd South Dunedin, Otago, New Zealand
- Cell Biotech Gimpo, South Korea
- Chr Hansen Horsholm, Denmark
- Centro Sperimentale del Latte s.r.l Zelo Buon Persico (Lodi), Italy
- Ch Deerland Enzymes Kennesaw, Georgia, USAinMax Medical Shanghai, China
- DANONE Paris, France
- Daflorn Sofia, Bulgaria
- Essential Formulas Farmers Branch, TX, USA
- Fit-bioceuticals Alexandria, New South Wales, Australia
- Futureceuticals Momence, IL, USA
- Invictus Pharmanutricao Sao Paulo, Brazil
- i-Health Cromwell, Connecticut, USA
- Ildong Pharmaceutical Seoul, Korea
- Jarrow Formulas Los Angeles, CA, USA
- KeVita Inc. Oxnard, CA, USA
- KGK Clinical Trial Centers London, ON, Canada
- Kibow Biotech Inc Newtown Square, PA, USA
- Lallemand Health Solutions Montreal, Canada
- Medlab Alexandria, Australia
- Kyolic, Wakunaga of America Mission Viejo, CA, USA
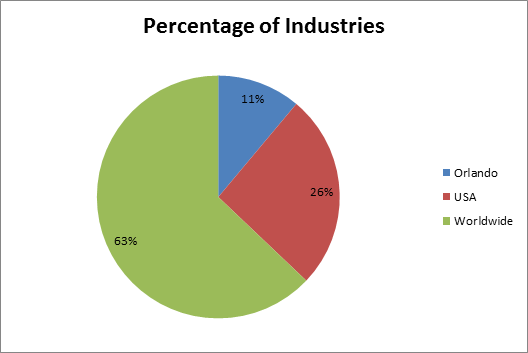
Figure-2: Industries Worldwide
Top Nutrition Universities in USA
- Baylor University
- Florida State
- McMaster University
- Michigan State
- New York University
- Ohio State
- Texas A&M
- University of British Columbia
- University of North Carolina at Chapel Hill
- Virginia Tech
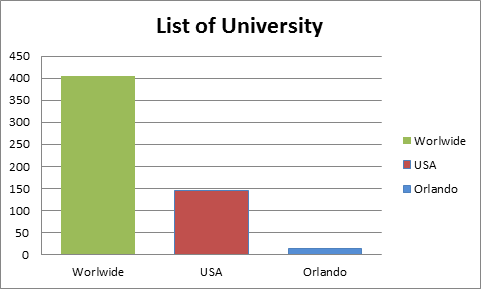
Figure-3: List of University
Target Audience
- Students
- Researchers
- Nutritionist
- Dietitian
- Physicians
- Public Health Professionals
- Sports Nutritionists
Industry-20%
Academia-60%
Others-20%
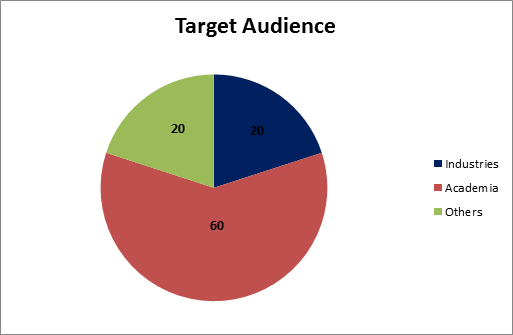
Figure-4: Target Audience
Glance at Market and Funding for Probiotics Research
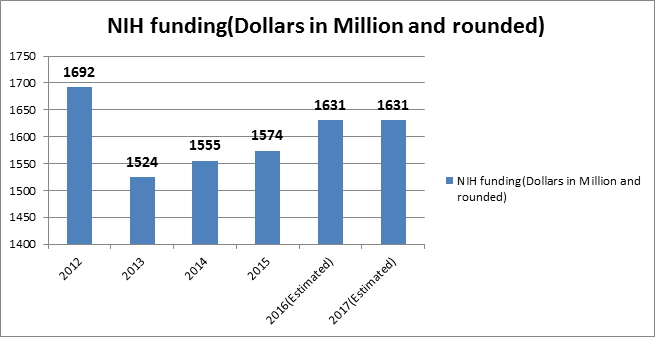
Figure-5: NIH Funding (Dollars in millions and rounded)
Projections: Growth by next 5-10 years:
The global market of probiotic ingredients, supplements, and foods reached nearly $23.1 billion in 2012. This market is expected to grow to nearly $27.1 billion in 2013 and $36.7 billion in 2018 with a compound annual growth rate (CAGR) of 6.2% over the five-year period from 2013 to 2018 and the market for probiotic ingredients is projected to reach USD 46.55 Billion by 2020 whereas global market for probiotics on the whole is expected to reach USD 52.34 billion by 2020, according to a new study by Grand View Research, Inc. Growing consumer awareness regarding gut health has pushed the demand for supplements and dietary products, which is expected to reflect in probiotic consumption over the forecast period.
TRACK 1. Probiotics and its impact on Human Health
Probiotics hold the key not just for better health and a stronger immune system, but also for healing digestive issues, mental health illness, and neurological disorders. The gut microenvironment has an effect on the nutrition, feed conversion and disease of the host, thereby maintaining the microbial ecology of the gut. During the periods of stress, illness or antibiotic treatment, the gut flora is often changed in favor of harmful bacteria that may cause diarrhea and loss of appetite. Overgrowth of the harmful bacteria and its subsequent invasion of the system lead to inflammatory, immunological, neurological and endocrinological problems. Induction of the growth of beneficial bacteria is one of the possible solutions to normalize the health conditions. This could be achieved by the supplementation of viable bacterial cells into the host. Probiotics can help to build up the beneficial bacterial flora in the intestine and completely exclude the pathogenic bacteria. These bacteria also release some enzymes which help in the digestion of the feed. A daily intake of 109-1010 colony forming units (CFU) viable cells has been shown to have positive effect on the host health. There are many microorganisms that could potentially function as probiotic, of which Lactobacillus and Bifidobacterium species are the most commonly used. Probiotics are live microorganisms thought to be beneficial to the host organism. According to the currently adopted definition by FAO/WHO, probiotics are live microorganisms, which when administered in adequate amounts confer a health benefit on the host.
TRACK 2. Probiotics in Functional Foods
Functional foods are similar in appearance to conventional foods; the former being consumed as part of the normal diet. In contrast to conventional foods, functional foods, however, have demonstrated physiological benefits and can reduce the risk of chronic disease beyond basic nutritional functions, including maintenance of gut health. When food is being cooked or prepared using "scientific intelligence" with or without knowledge of how or why it is being used, the food is called "functional food". Thus, functional food provides the body with the required amount of vitamins, fats, proteins, carbohydrates, etc., needed for its healthy survival.
TRACK 3. Pediatrics Nutrition and its benefits
There is no question that breast milk is the best form of food for newborns and infants, and should be the number one choice for all mothers. However, some mothers are unable to breastfeed, which is why it’s important they have access to safe and nutritious alternatives. When it comes to infant formula, the aim and intent of the World Health Organisation (WHO) International Code of Marketing Breast Milk Substitutes is fully supported. Breast milk substitutes are recognised by the WHO as a safe and nutritious alternative to breast milk for infants whose mothers cannot, or choose not to, breastfeed. Probiotics are supplements or foods that contain viable microorganisms that cause alterations of the microflora of the host. Use of probiotics has been shown to be modestly effective in randomized clinical trials (RCTs) in (1) Treatment of acute viral gastroenteritis in healthy children (2) Prevention of antibiotic-associated diarrhea in healthy children. There is some evidence that probiotics prevent necrotizing enterocolitis in very low birth weight infants (birth weight between 1000 and 1500 g). The results of RCTs in which probiotics were used to treat childhood Helicobacter pylori gastritis, irritable bowel syndrome, chronic ulcerative colitis, and infantile colic, as well as in preventing childhood atopy, are also encouraging the use of probiotics for infants.
TRACK 4. Animal Probiotics
In human nutrition, lactobacilli and bifidobacteria are frequently included in yoghurts and other milk products. However, due to their poor stability during storage, their application in animal nutrition is rather limited. Probiotic feed additives generally consist of one single strain or a combination of several strains of bacteria, Bacillus spores or yeasts species (multi-strain). Preparations authorised for use in animal nutrition include different strains of Enterococcus, Bacillus, Lactobacillus in the European Union , Pediococcus or Saccharomyces. The benefit of probiotics with respect to health status and performance is expected to be highest in young animals such as piglets, newly-hatched chickens or calves, because these animals have not yet developed a stable gut microflora. Moreover, when animals undergo therapeutic treatment of diseases with antibiotics, the gut microflora is generally decimated. Therefore, administration of probiotics after antibiotic treatment assists in re-establishing a beneficial gut microflora to prevent the host from recurrent pathogenic colonisation.
Silage inoculants is another important factor of animal nutrition which is helps in providing aid for the process of fermentation by reducing the forage quality loss and helps in maintaining high quality feed and palatability that will also lead to improved animal performance thereby, extending the shelf life of silages. Spoilage microorganisms such as bacteria, yeasts, and molds readily grow on crops going into a silo, causing losses in dry matter and nutrient quality. Having an oxygen-free (anaerobic) environment and a low pH in the silo can prevent these organisms from growing. These spoilage microorganisms are also the culprits in poor fermentation. Efficient fermentation will conserve dry matter to boost feed value. Homofermentative lactic acid bacteria, such as Lactobacillus plantarum, Enterococcus faecium, and some species of Pediococci can improve the initial fermentation process by speeding up the production of lactic acid and limiting the production of unnecessary end products that may lower the efficiency of fermentation. Creating a desirable environment in the silo (low pH) can reduce protein degradation and prevent the growth of several microbes in the silage like Enterobacteria, Clostridia and mold
TRACK 5. Delivery Vehicles
Probiotics are live microorganisms which confer a health benefit on the host when administered in an adequate amount. Over the past decades, the market size of probiotics has greatly increased as modern consumer concern about health-promoting effect of nutraceuticals. Since probiotic-containing products in general do not require Food and Drug Administration approval, they are commonly available in the market in various food formats such as fermented milk, cheese, yogurt and juice. In recent years, probiotics have been extensively studied as a treatment option of various diseases such as obesity, diabetes, cancer, human immunodeficiency virus infection, irritable bowel syndrome. For probiotics to exert beneficial activities, a sufficient amount of probiotics should be alive and functionally active at the site of action as well as in a product. Probiotics are recommended to be present at a minimum level of 6 log colony forming unit (CFU)/g in a food product or 7 log CFU/g at the point of delivery. Due to the vulnerability of probiotics to harsh conditions during manufacturing, storage and passage through the gastrointestinal (GI) tract, however, it is difficult that viable probiotics successfully exert beneficial activities. During manufacturing and/or storage, the viability of probiotics can be negatively affected by several factors such as temperature, water activity and other food ingredients. Specifically, high temperature during manufacturing processes is a main reason for reduced viability because most probiotics have low thermo-resistance. Maintaining viability in the stomach is another difficult task for probiotics to reach the target site because most of probiotics die or lose their functionality at acidic conditions. Next, survived probiotics should be released at the target site of action which is usually small or large intestine. Therefore, an ideal probiotic delivery system should protect probiotics from adverse conditions during fabrication and storage and in the acidic gastric environment so that the sufficient amount of probiotics is available in the site of action
TRACK 6. Mechanism of Action of Probiotics
Our gut contains both beneficial and harmful bacteria. Digestive experts agree that the balance of gut flora should be approximately 85 percent good bacteria and 15 percent bad bacteria. If this ratio gets out of balance, the condition is known as dysbiosis, which means there is an imbalance of too much of a certain type of fungus, yeast or bacteria that is affecting the body in a negative way. By consuming certain types of probiotics foods and supplements we can help bring these ratios back into balance. The efficacy of a probiotic effect often depends on the mechanism by which they exert their activity. By and large, to treat a disease, the probiotics follow a set of mechanisms, which is discussed in this review. The effective performance of the probiotic depends on their strong adherence and colonization of the human gut, which in turn improves the host immune system. The mechanism of adherence is still under investigation, but Lactobacillus plantarum 299v has been shown to exhibit a mannose specific adhesion by which it can adhere to human colonic cells. Once the probiotic adheres to the cell, various biological activities take place, which primarily include the release of cytokine’s and chemokine’s. These then exert their secondary activity such as stimulation of mucosal and systemic host immunity
TRACK 7. Probiotic-Derived Factors: Probioceuticals
They are novel, clinically proven and safe molecules present in the market, whose health claims are approved by various international and/or Indian regulatory authorities. Probiotic-derived factors have been described as capable of exerting probiotic activities through probiotic mechanism of actions Although there are many bacteria-derived products capable of inducing a health benefit, the concept of probiotic is only attributed to microorganisms administered as viable forms, providing the opportunity for a symbiotic relationship between the host, and resident, or in-transit, microorganisms. Secreted probiotic factors, such as reuterin from Lactobacillus reuteri, have been reported to inhibit adhesion and viability of known enteric pathogens, suggesting that probiotic supernatants could be a rich source of new antipathogenic compounds.
TRACK 8. Safety Considerations
People come into contact with probiotics used in animal nutrition in two ways, either as workers in the production of premixes and compound feeds, or as farmers during feeding. In both cases there are no hazards for the users. Comprehensive studies have shown that direct contact of the registered probiotic products with skin, mouth and nose do not compromise human health. In model trials it has been established that even long-term or increased exposure do not constitute a risk to health. In general, the microorganisms approved for animal nutrition have a very good safety record. Even in cases of overdoses of more than a thousand times the recommended levels in feed, there are no signs of dysbiosis in the gastrointestinal tract. Therefore, probiotics do not constitute any health hazard for the animal. Since they are not transferred from the intestine into the body of the animal, they do not affect any metabolic processes, nor do they have any negative impact on the animal. Having exerted their effect in the digestive tract, the probiotic reaches the exit of the intestine in the digesta, together with other intestinal microorganisms. On their way along the digestive tract the majority of the probiotic bacteria die off, since their growth and proliferation is severely restricted by competition from other microorganisms present in the large intestine. The development of yeasts is also suppressed by a lack of oxygen. The probiotics are already partly broken down and digested like other organic nutrients in the intestine so that only a small proportion is excreted viable in the faeces and survives in the manure to reach fields and grassland. Evidence of the harmlessness of the probiotic to the environment is one important subject for its registration. In general, any negative impact is highly unlikely since all these microorganisms are derived from nature.
TRACK 9. Regulatory Status of Probiotic Foods
Any substance that is reasonably expected to become a component of food is a food additive that is subjected to premarket approval by FDA, so if a probiotic is intended for use as a drug, then it must undergo the regulatory process as a drug, which is similar to that of any new therapeutic agent. An Investigational New Drug application must be submitted and authorized by FDA before an investigational or biological product can be administered to humans. The probiotic drug must be proven safe and effective for its intended use before marketing. If a probiotic is intended for use as a dietary supplement, it is placed under the umbrella of “foods,” and as such is regulated by FDA’s Center for Food Safety and Applied Nutrition. In contrast to drugs, dietary supplements do not need FDA approval before being marketed. However, manufacturers need to notify FDA before marketing a product. According to Dietary Supplement Health and Education Act, 1994 (DSHEA), the manufacturer is responsible for determining that the dietary supplements that it manufactures or distributes are safe and that any representations or claims made about them are substantiated by adequate evidence to show that they are not false or misleading; the manufacturers need not provide FDA with evidence that substantiates the safety or purported benefits of their products, either before or after marketing. The law allows that in addition to nutrient content claims, manufacturers of dietary supplements may make structure/function or health claims for their products. For a structure/function claim, FDA requires that manufacturers’ substantiation is accepted by experts in the field and that the claim is truthful and not misleading. The data substantiating structure/function claims need not be publicly available and need not be disclosed. In 2001, in an attempt to standardize the requirements needed to make health claims regarding probiotic agents, the Joint Food and Agriculture Organization of the United Nations/World Health Organization Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics developed guidelines for evaluating probiotics in food that could lead to the substantiation of health claims. The Consultation recommends that specific health claims on labeling material on probiotic food items be allowed when sufficient scientific evidence is available and that the product manufacturer take responsibility for ensuring that an independent third party reviews and evaluates the scientific evidence
TRACK 10. Probiotics Applications and Challenges
In addition to restoring the microbial balance of the Gastrointestinal Tract (GIT), products containing probiotic organisms, are also claimed to propose to have several dietary and therapeutic or functional benefits. Preventive and therapeutic effects of these agents have been observed in the lactose intolerance, constipation, hypercholesterolemia, lactose intolerance, virginities, intestinal infections and various cancers such as breast cancer, bladder cancer, colon cancer, liver cancer. But there are many challenges in the development of a probiotic food product such as strain selection, inoculation, growth and survival during processing, viability and functionality during storage, assessment of the viable counts of the probiotic strains particularly when multiple probiotic strains are added and when there are also starter cultures added, and the effects on sensory properties.
TRACK 11. Future of Probiotics visions and opportunities
Probiotic microorganisms can shape the immune system both at the local and systemic level which will allow future probiotics as treatments for many diseases. Probiotics seem to have promising role in shortening duration of infections or decreasing susceptibility to the pathogens. Use of the different strains, dosage, duration of treatment and smaller size of the trials makes interpretation of the available data more difficult. Current evidence also indicates that probiotic effects are strain-specific, they do not act through the same mechanisms nor are all probiotics indicated for the same health conditions. It is currently unknown whether there are optimal probiotic species, doses, and/or formulations. Although the data with probiotics are still far too weak to convince clinicians, the concept is fascinating, and further studies would be more than welcome. Nanotechnology of probiotics is an area of emerging interest and opens up whole new possibilities for the probiotics applications. Their applications to the agriculture and food sector are relatively recent compared with their use in drug delivery and pharmaceuticals. The basic of probiotic nanotechnology applications is currently in the development of nano-encapsulated probiotics.
Conference Highlights
- Probiotics in Functional Foods
- Pediatrics Nutrition and its benefits
- Animal Probiotics
- Mechanism of Action of Probiotics
- Probiotic-Derived Factors: Probioceuticals
- Safety Considerations
- Regulatory Status of Probiotic Foods
- Probiotics Applications and Challenges
- Future of Probiotics visions and opportunities
- Probiotics and its impact on Human Health
- Delivery Vehicles
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | November 14-16, 2016 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Probiotics & Health
- Journal of Nutrition & Food Sciences
- Journal of Microbial & Biochemical Technology
Abstracts will be provided with Digital Object Identifier by